lewis dot structure of propane|C3H8 (Propane) Lewis Structure in 6 Steps (With Images) : iloilo We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its .
10 yearsExperience massage therapist,succeeded completed the competency required under the Philippines TVET QUALFICATION AND CERTIFICATION(NC||- TESDA CERTIFICATE,QUALIFIED IN:SWEDISH MASSAGE,CHAIR MASSAGE,FOOT MASSAGE,PREGNANT SIDE/ LYING MASSAGE,BODY STRETCHING,BELLY .
PH0 · Propane C3H8 Lewis Dot Structure
PH1 · Lewis Structure of C3H8 (Propane) (In 6 Simple Steps)
PH2 · Lewis Dot Structures
PH3 · How to Draw the Lewis Structure for C3H8 (Propane)
PH4 · How to Draw a Lewis Structure
PH5 · C3H8 Lewis structure
PH6 · C3H8 Lewis Structure
PH7 · C3H8 (Propane) Lewis Structure in 6 Steps (With Images)
PH8 · 9.3: Drawing Lewis Structures
PH9 · 7.3 Lewis Symbols and Structures
Estrazioni del Gioco del Lotto degli ultimi 60 giorni. Per riscuotere una vincita hai 60 giorni di tempo dalla pubblicazione dei numeri nel Bollettino Ufficiale che deve essere affisso in ricevitoria. Controlla qui i numeri appena estratti e l'archivio delle ultime estrazioni per sapere se hai vinto. Estrazione n. 141 del 03/09/2024.
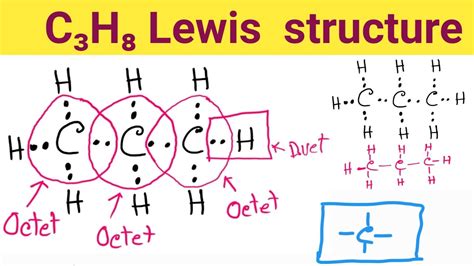
lewis dot structure of propane*******A video explanation of how to draw the Lewis Dot Structure for Propane, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, .Transcript: This is the C3H8 Lewis structure: Propane. We have 4 valence electrons for Carbon--we have 3 Carbons. We have 1 valence electron for Hydrogen--8 Hydrogens. Add them up, . C 3 H 8 (propane) has three carbon atoms and eight hydrogen atoms. In the C 3 H 8 Lewis structure, there are two single bonds between the three carbon atoms. The left .
C3H8 (Propane) lewis structure has a single bond between the Carbon-Carbon atoms (C) as well as between the Carbon atom (C) and Hydrogen atom (H). The three Carbon .
Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the .We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its .
To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one . A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. The diagram is also called a Lewis dot diagram, Lewis dot formula, or electron . 118K views 10 years ago. A step-by-step explanation of how to draw the C3H8 Lewis Dot Structure (Propane). For the C3H8 structure use the periodic table to find the total number of valence.
A video explanation of how to draw the Lewis Dot Structure for Propane, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, Shape, and Bond Angles.Transcript: This is the C3H8 Lewis structure: Propane. We have 4 valence electrons for Carbon--we have 3 Carbons. We have 1 valence electron for Hydrogen--8 Hydrogens. Add them up, we get a total of 20 valence electrons for the C3H8 Lewis structure. We'll put the 3 Carbons in a row and the Hydrogens will go around them. Lewis structure of C3H8 (or Propane) contains single bonds between Carbon-Carbon atoms as well as between Carbon-Hydrogen atoms. The three Carbon atoms (C) are at the center and they are surrounded by Hydrogen atoms (H). Let’s draw and understand this lewis dot structure step by step. C 3 H 8 (propane) has three carbon atoms and eight hydrogen atoms. In the C 3 H 8 Lewis structure, there are two single bonds between the three carbon atoms. The left carbon and right carbon are attached with three hydrogen atoms, and the center carbon is attached with two hydrogen atoms. C3H8 (Propane) lewis structure has a single bond between the Carbon-Carbon atoms (C) as well as between the Carbon atom (C) and Hydrogen atom (H). The three Carbon atoms (C) are at the center and they are surrounded by the Hydrogen atoms (H).
Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule.We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of .lewis dot structure of propane C3H8 (Propane) Lewis Structure in 6 Steps (With Images) To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron – never seven.
lewis dot structure of propane A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. The diagram is also called a Lewis dot diagram, Lewis dot formula, or electron dot diagram. 118K views 10 years ago. A step-by-step explanation of how to draw the C3H8 Lewis Dot Structure (Propane). For the C3H8 structure use the periodic table to find the total number of valence. A video explanation of how to draw the Lewis Dot Structure for Propane, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, Shape, and Bond Angles.C3H8 (Propane) Lewis Structure in 6 Steps (With Images)Transcript: This is the C3H8 Lewis structure: Propane. We have 4 valence electrons for Carbon--we have 3 Carbons. We have 1 valence electron for Hydrogen--8 Hydrogens. Add them up, we get a total of 20 valence electrons for the C3H8 Lewis structure. We'll put the 3 Carbons in a row and the Hydrogens will go around them.

Lewis structure of C3H8 (or Propane) contains single bonds between Carbon-Carbon atoms as well as between Carbon-Hydrogen atoms. The three Carbon atoms (C) are at the center and they are surrounded by Hydrogen atoms (H). Let’s draw and understand this lewis dot structure step by step.
C 3 H 8 (propane) has three carbon atoms and eight hydrogen atoms. In the C 3 H 8 Lewis structure, there are two single bonds between the three carbon atoms. The left carbon and right carbon are attached with three hydrogen atoms, and the center carbon is attached with two hydrogen atoms.
C3H8 (Propane) lewis structure has a single bond between the Carbon-Carbon atoms (C) as well as between the Carbon atom (C) and Hydrogen atom (H). The three Carbon atoms (C) are at the center and they are surrounded by the Hydrogen atoms (H).
Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule.We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of .
10.0.0.1 Piso WiFi: A Piso WiFi setup has become increasingly popular in internet connectivity and wireless networks. Whether you’re a business owner looking to provide affordable internet access to your customers or an individual seeking a convenient way to share your internet connection, a Piso WiFi system can be a great solution.. To .
lewis dot structure of propane|C3H8 (Propane) Lewis Structure in 6 Steps (With Images)